You can do everything right in a clinical trial, but accurate and effective communication will determine your final success.
Researchers collaborate on clinical trials globally—across different languages, dialects, and cultural backgrounds. As researchers conduct medical trials to innovate solutions and medical interventions, they need to connect with peers and participants with clarity.
The better researchers can communicate with participants, patients, and the medical community throughout translated clinical trial materials, the better their research and results.
Clinical trial translation doesn't have to be costly, slow, error-prone, or complex thanks to AI-powered human translations. You can convey your trial protocol and results across language barriers with robust technology and the native-speaking expertise of professional translators.
In this article, we’ll review the role translations play in medical research, what true translation accuracy looks like, and how implementing modern translation tools can power improved research outcomes.
The critical role of medical translation in clinical trials
Precision matters in clinical trials, especially when many investments, grants, and stakeholders are involved.
Accurate translation of medical research can safeguard against many liabilities, but more importantly, lives often depend on scientific and healthcare advancements. There's no room for misunderstanding.
Mistranslations can:
- Result in misdiagnosis
- Reduce the quality of participant or patient care and comfort
- Negatively influence patient safety or study outcomes
- Impact the development of innovative technologies and treatments
Researchers should be able to trust translations of their team communication, patient and participant records, medical device research, and trial results.
The good news is that a better translation can resolve these obstacles. But what are the attributes of a truly accurate translation?
Defining accurate translations for global clinical trials
 Localization, or translations for local audiences and cultures, takes multiple factors into consideration.
Localization, or translations for local audiences and cultures, takes multiple factors into consideration.
Translations aren’t simply translating clinical trial content word-for-word. There's considerable nuance involved, especially in the medical field.
Add to this the cultural complexities of various people, groups, and languages. Expert linguists know the value of accurate translation, not just in a word-by-word context but also in depicting the author's intended message.
For example, a medical form prompting participants to voice discomfort and side effects may require a localized translation, considering how a culture may perceive the content.
If a people group is more reluctant to communicate an issue, the form’s language may need to be softer and more welcoming. An opposite cultural style may require more direct and upfront communication.
A strong translation considers both the literal translation and how each target audience may perceive the information in the context of their cultural understanding.
In Dr. Susana Valdez’s study published by the University of Lisbon on translation processes within the biomedical research field, she states:
"Translators think that revisers and health professionals value translations that are both source- and target-oriented, based on (source-oriented) beliefs such as accuracy and fidelity towards the author's intended message, and (target-oriented) beliefs regarding the desirability of fluent and natural-sounding texts that are linguistically correct and terminologically accurate and consistent."
In summary, accurate translations combine literal translation, the author's intent, and the audience's perception. Such accuracy is invaluable across a wide variety of clinical trial materials.
Critical use cases for clinical trial translation
Translation accuracy is vital any time your research team communicates with a multilingual peer during trials or interacts with a participant who speaks a different language. Below are a few key areas that often require daily translation accuracy:
1. Forms, files, and labeling
One immediate example is participant consent forms. As a leader within your clinical study, you must ensure that participants fully understand your trial's purpose, risks, and context. Failure to convey your intended message can risk participant comprehension, present ethical dilemmas, and incur legal roadblocks.
Other clinical trial documents include patient diary logs, questionnaires, recruitment materials, and other essential elements for collecting participant data.
2. Written communication and collaboration
When you conduct your clinical trial research site or study in another language or another country, you must navigate regulatory translation requirements, ethics committees, and institutional review boards.
You must also accurately follow the documentation and publication process of medical trials. Researchers and pharmaceutical companies can better communicate clinical trial protocols when they accurately convey objectives, methodologies, and considerations to a global medical community.
Better translation ensures that participants and researchers follow all trial procedures correctly, regardless of the location, context, or languages involved. It also supports collaboration between researchers and members of the medical community. They allow each person to easily communicate with clarity and specificity.
Clinical trial settings may also require back translations. That’s when an individual or AI uses a translated message and transforms it back to the source language. The final result should have the same meaning as the original text. Key leaders in governing bodies often require back translations to understand the trial’s study, project, and origin.
3. Training materials
Clinical trials involve providing education and training materials to many different roles and stakeholders, such as staff and trial participants. Translations ease communication with different people groups and provide consistent training, clear guidelines on procedures and considerations, and supporting materials. This can streamline operations for a successful trial.
4. Participant experience and care
Finally, clinical trial leaders should prioritize the participant experience to ensure their safety and comfort. Whether testing medical devices, life-saving drugs, or procedures, it's important to understand your participants to provide the best care and fulfill their pressing needs.
Your research teams want to connect with each participant and patient on the same level. AI-powered human translation can help them do so affordably and efficiently.
AI-powered human translation technology
Traditional translation processes are costly, slow, and often inaccurate. But you don't have to rely on these inefficient translation solutions for your clinical trials.
A translation company solution like Smartling offers the technology and language services you need to produce accurate, affordable, and speedy translations.
For example, researchers or translation teams can input billions of words of source text and get translated content in seconds through the Neural Machine Translation (NMT) Hub. Machine translations (MTs) produce super-fast, high-quality translation results, so you can translate quickly and in large quantities.
The best approach combines MT with expert translators. These translators aren't your average linguists. They’re specialized, native-speaking translators who understand the target language and nuances within its culture. They can use a translation management system (TMS) for their workflow and verify accuracy for the cultural complexities of a multilingual audience—whether that's a co-worker, a participant in a study, or the scientific community.
This AI-powered human approach leverages the best of both worlds: fast and affordable translations with pinpoint cultural accuracy.
The benefits of AI-powered human experts
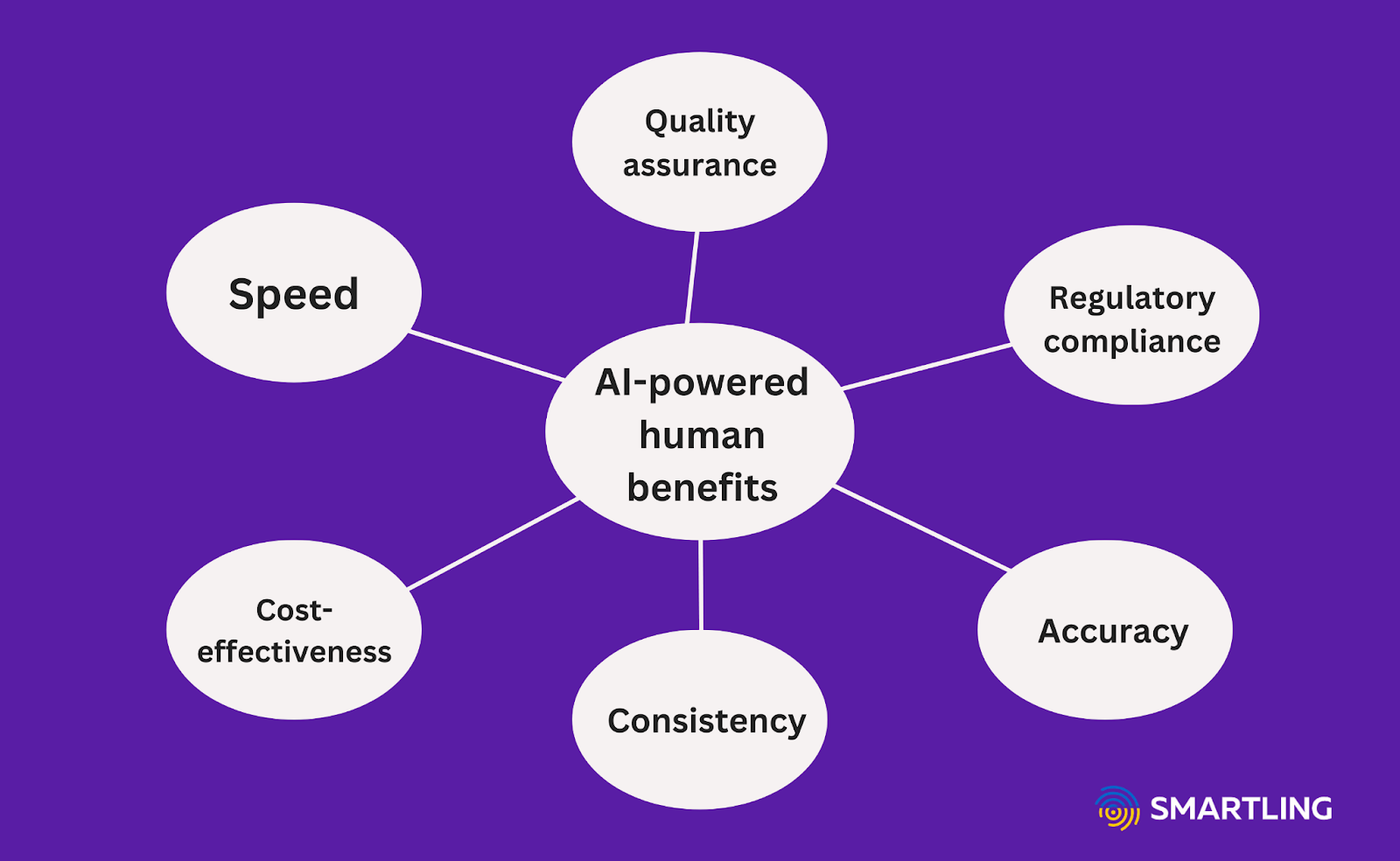 The AI-powered human translation approach offers the best strategy for accurate clinical trial translations.
The AI-powered human translation approach offers the best strategy for accurate clinical trial translations.
The combined approach of the latest technology and high-quality human translators offers clear advantages for clinical trials:
- Speed: Researchers can worry less about clinical trial delays due to slow translational processes.
- Cost-effectiveness: The translation workflow is highly affordable and efficient, thanks to machine technology and high-performing linguists. This saves researchers resources, allowing them to spend more time on their studies.
- Consistency: Thanks to technology like translation memory and glossaries, experts can log specific terms, phrases, and cultural preferences for automatic application during MT. This ensures a consistent clinical trial communication standard and saves time for future translations.
- Accuracy: Between AI technologies and local linguists, researchers can expect better translations that communicate efficiently to all participants, relevant medical communities, and fellow researchers.
- Regulatory compliance: Clinical trials involve many different legal and regulatory considerations, especially within global settings. Local human translators can help you navigate these translations and contexts, as well as the legal and ethical challenges for each country or region.
- Quality assurance: With machine learning standards and measurements like Multidimensional Quality Metrics (MQM), you can ensure accurate translations. Expert translators can also review AI output for localization and revisions when necessary.
The key is finding the right tool that unites these technologies and linguist services all in one place.
Why Smartling is the top choice for clinical trial translation
 Clinical research organizations can choose Smartling for clinical trial translations. (Source)
Clinical research organizations can choose Smartling for clinical trial translations. (Source)
When you lead a clinical trial, balancing many cultures and languages can affect your work. Translations go beyond converting English to Spanish, or from any language to another, word-for-word. They involve complex cultures, dialects, and diverse people groups. Communication is critical, so finding an accurate, affordable, and fast translation solution is crucial.
Smartling offers the tools to efficiently translate billions of research texts through AI-powered NMT technology, manage translation workflows within a sophisticated TMS, and account for every cultural nuance with localized human clinical trial translation services.
Smartling’s AI-powered human approach can help mitigate language barriers and avoid misdiagnoses and research delays in your clinical trials.
Book a meeting today to see how you can improve your clinical trials with expert translation services and tools.








